Search results
Search for "inclusion complexes" in Full Text gives 128 result(s) in Beilstein Journal of Organic Chemistry.
Spatial arrangements of cyclodextrin host–guest complexes in solution studied by 13C NMR and molecular modelling
Beilstein J. Org. Chem. 2024, 20, 331–335, doi:10.3762/bjoc.20.33
- dispersion forces and hydrogen bonding between the cyclodextrin (CD) unit and the guest molecule. Determination of the ee of chiral guests was achieved by observing the splitting of 1H NMR signals of the achiral host upon formation of diastereomeric inclusion complexes [18][19]. Shifting the H-3 and H-5
Cyclodextrins permeabilize DPPC liposome membranes: a focus on cholesterol content, cyclodextrin type, and concentration
Beilstein J. Org. Chem. 2023, 19, 1570–1579, doi:10.3762/bjoc.19.115

- between HP-β-CD and DPPC rather than with CHOL which explains the obtained results. As for SBE-β-CD, it was reported that charged CDs could not interact with CHOL molecules and form inclusion complexes due to charge repulsion [26]. This could explain the results obtained for these two CDs. Hence, HP-β-CD
- combined delivery system “drug-in-cyclodextrin-in-liposomes” (DCL) where CD–drug inclusion complexes are in contact with the membrane. The choice of CD in such a system does not only depend on the drug affinity towards the CD cavity, but should also take into consideration the affinity of the selected CD
A fluorescent probe for detection of Hg2+ ions constructed by tetramethyl cucurbit[6]uril and 1,2-bis(4-pyridyl)ethene
Beilstein J. Org. Chem. 2023, 19, 864–872, doi:10.3762/bjoc.19.63

- form various host–guest inclusion complexes [16][17][18][19][20], which have broad application prospects in supramolecular catalysis [21][22][23], molecular recognition [24][25], and drug delivery [26][27]. In recent years, in the field of supramolecular chemistry, the detection of analytes based on
- Figure 4c, the hydrogen atoms of G and the carbonyl oxygen of TMeQ[6] form C–H22···O1, C–H26···O1, C–H25···O4 and C–H27···O4 hydrogen bonds with bond distances of 2.370, 2.474, 2.564 and 2.685 Å, respectively. These interactions contribute to the formation of stable inclusion complexes. Figure 4d is a
Discrimination of β-cyclodextrin/hazelnut (Corylus avellana L.) oil/flavonoid glycoside and flavonolignan ternary complexes by Fourier-transform infrared spectroscopy coupled with principal component analysis
Beilstein J. Org. Chem. 2023, 19, 380–398, doi:10.3762/bjoc.19.30
- compounds [5]. The resulting supramolecular inclusion complexes provide enhanced water solubility and bioavailability/bioaccessibility of the nanoencapsulated bioactive compounds, higher oxidative and thermal stability or photostability of labile compounds, and their controlled release [6][7]. Vegetable oil
- thermal/oxidative stability of ternary complexes is similar to β-CD hydrate, as was evaluated by TG and DSC. Moreover, the formation of the molecular inclusion complexes is supported by thermal analysis (partial replacing of the hydration water by biologically active molecules and disappearance of the DSC
CuAAC-inspired synthesis of 1,2,3-triazole-bridged porphyrin conjugates: an overview
Beilstein J. Org. Chem. 2023, 19, 349–379, doi:10.3762/bjoc.19.29
- . Furthermore, these two structurally similar porphyrins 166a,b were used as hosts for the intermolecular formation of inclusion complexes with tetrasodium tetraphenylporphyrintetrasulfonate 132 in aqueous solution, leading to the constructions of two different nanoarchitectures with alternating porphyrin and
Insight into oral amphiphilic cyclodextrin nanoparticles for colorectal cancer: comprehensive mathematical model of drug release kinetic studies and antitumoral efficacy in 3D spheroid colon tumors
Beilstein J. Org. Chem. 2023, 19, 139–157, doi:10.3762/bjoc.19.14

- having hydrophobic cavities and hydrophilic exterior surfaces, CDs are widely used in the pharmaceutical field to form inclusion complexes mostly with nonpolar molecules in their cavities [22]. In addition, CDs also offer several advantages for colonic drug delivery, because CDs are broken down by the
Preparation of β-cyclodextrin/polysaccharide foams using saponin
Beilstein J. Org. Chem. 2023, 19, 78–88, doi:10.3762/bjoc.19.7

- Max Petitjean Jose Ramon Isasi Department of Chemistry. University of Navarra. 31080 Pamplona, Spain 10.3762/bjoc.19.7 Abstract Cyclodextrins, cyclic oligosaccharides with a hydrophobic cavity that form inclusion complexes with nonpolar molecules, can be used to functionalize other
Inclusion complexes of the steroid hormones 17β-estradiol and progesterone with β- and γ-cyclodextrin hosts: syntheses, X-ray structures, thermal analyses and API solubility enhancements
Beilstein J. Org. Chem. 2022, 18, 1749–1762, doi:10.3762/bjoc.18.184
- ingredients (APIs) is necessary to render them bioavailable. This study addresses the poor solubility of two potent steroid hormones, 17β-estradiol (BES) and progesterone (PRO), via their complexation with two water-soluble native cyclodextrins (CDs) namely β-CD and γ-CD. The hydrated inclusion complexes β
- host–guest stoichiometries and water contents of the four hydrated inclusion complexes enabled accurate assignment of the chemical formulae of these ternary systems. Predicted electron counts for the complexed molecules BES and PRO correlated reasonably well with the complex compositions indicated by
- pharmaceutical industry for many decades. However, the complexation of APIs with cyclodextrins (cyclic oligosaccharides) resulting in the formation of inclusion complexes, has proven to be a versatile technology for overcoming not only the poor aqueous solubility of APIs, but also unfavourable properties such as
1,4,6,10-Tetraazaadamantanes (TAADs) with N-amino groups: synthesis and formation of boron chelates and host–guest complexes
Beilstein J. Org. Chem. 2022, 18, 1424–1434, doi:10.3762/bjoc.18.148
- hydrazone groups. The use of N-TAAD derivatives as potential ligands and receptors was showcased through forming boron chelates and host–guest complexes with water and simple alcohols. Keywords: azaadamantanes; cyclotrimerization; hydrazones; inclusion complexes; molecular recognition; Introduction
- and carbamate groups form inclusion complexes with water or methanol (Figure 3a–d). In the crystal state, a common hydrogen-bonded motif is observed, in which the guest molecule is located in a pocket created by amide/carbamate groups and the triazinane ring of the 3N-TAAD structure that is well
- ). Such symmetrization can be explained by a pre-organization of N-amido groups through the coordination of water/alcohol leading to the host–guest complex observed in the X-ray. The formation of the inclusion complexes H2O@TAADs 4a and MeOH@4c·H+ was additionally explored by DFT calculations at the
Cyclodextrin-based Schiff base pro-fragrances: Synthesis and release studies
Beilstein J. Org. Chem. 2022, 18, 1346–1354, doi:10.3762/bjoc.18.140
- hydrophilic outer surface and hydrophobic cavity. This cavity can encapsulate another lipophilic guest molecule and thus form an inclusion complex [3][4]. This phenomenon is reversible and leads to an equilibrium between encapsulated and free guest. A staggering number of inclusion complexes of CDs with
- easiest accessibility; besides, β-CD forms usually the strongest inclusion complexes compared to α-CD and γ-CD. The amino-β-CD was prepared according to published procedures [25][26][27]. Common commercially available volatile aldehydes 2a–j (Figure 2) were chosen as the aldehyde reactants. Some of the
- that CD-based pro-fragrances combine a chemical barrier, as the aldehydes are linked to the CD via imine bond, and a physicochemical (supramolecular) barrier, as we established that aldehydes formed inclusion complexes in aqueous solution with β-CD. We confirmed from the NMR kinetic study of the imine
Using multiple self-sorting for switching functions in discrete multicomponent systems
Beilstein J. Org. Chem. 2020, 16, 2831–2853, doi:10.3762/bjoc.16.233

- . Interestingly, the fullerene C60 was not taken up as a guest by the tetramer (6)4 in chlorinated solvents. For a more defined self-sorting, the authors switched the solvent from CDCl3 to [d8]-toluene. Now, a 2-fold completive self-sorting delivered the homoleptic inclusion complexes [(5)4(C60)] and [(6)4(C60
Particle size effect in the mechanically assisted synthesis of β-cyclodextrin mesitylene sulfonate
Beilstein J. Org. Chem. 2020, 16, 2598–2606, doi:10.3762/bjoc.16.211
- , 7, and 8 α-ᴅ-glucopyranoside units, respectively. CDs are recognized as effective excipients in the formulation of numerous drugs [7][8][9][10]. Upon grinding, CDs form inclusion complexes with the drugs in the solid state, resulting in a significantly faster dissolution rate and increased
- greatly improved. However, it was not clear whether the increase in reactivity was only a consequence of the formation of inclusion complexes, or whether the grinding process was also involved, and if so to what extent. Accordingly, the quantification of the grinding contribution in the reactivity was
[3 + 2] Cycloaddition with photogenerated azomethine ylides in β-cyclodextrin
Beilstein J. Org. Chem. 2020, 16, 1296–1304, doi:10.3762/bjoc.16.110
- Zagreb, Croatia 10.3762/bjoc.16.110 Abstract Stability constants for the inclusion complexes of cyclohexylphthalimide 2 and adamantylphthalimide 3 with β-cyclodextrin (β-CD) were determined by 1H NMR titration, K = 190 ± 50 M−1, and K = 2600 ± 600 M−1, respectively. Photochemical reactivity of the
- inclusion complexes 2@β-CD and 3@β-CD was investigated, and we found out that β-CD does not affect the decarboxylation efficiency, while it affects the subsequent photochemical H-abstraction, resulting in different product distribution upon irradiation in the presence of β-CD. The formation of ternary
- systems for the control of photoreactivity. Keywords: [3 + 2] cycloadditions; β-cyclodextrin; inclusion complexes; photochemistry; phthalimides; Introduction Cycloadditions are highly useful reactions in organic synthesis providing complex cyclic structures from easily available precursors [1][2]. Among
The interaction between cucurbit[8]uril and baicalein and the effect on baicalein properties
Beilstein J. Org. Chem. 2020, 16, 71–77, doi:10.3762/bjoc.16.9
- suggested that Q[8] included cycle B and part of cycle C of BALE into its cavity. To quantitatively determine the ratio of the host–guest inclusion complexes formed from Q[8] and the guest, the UV spectra of different aqueous solutions containing a fixed concentration of the guest (20 μmol·L−1) and variable
Anion-driven encapsulation of cationic guests inside pyridine[4]arene dimers
Beilstein J. Org. Chem. 2019, 15, 2486–2492, doi:10.3762/bjoc.15.241

- could not be verified and the anions were assumed to interact with the pyridinearene cavity. Inclusion complexes of anions within pyridinearene dimers were also theoretically studied by DFT calculations, but using a truncated pyridinearene dimer model [8]. However, previous studies have also shown that
Reversible switching of arylazopyrazole within a metal–organic cage
Beilstein J. Org. Chem. 2019, 15, 2398–2407, doi:10.3762/bjoc.15.232

- green light and resulted in the initial 1:2 cage/E-arylazopyrazole complex. This back-isomerization reaction also proceeded in the dark, with a rate significantly higher than in the absence of the cage. Keywords: arylazopyrazoles; coordination cages; inclusion complexes; molecular switches
- on the prototypical arylazopyrazole 1 [35] (Scheme 1) and a previously reported [48] metal–organic cage 2 (see Figure 1). We have recently demonstrated that various azobenzenes formed 2:1 inclusion complexes with 2 [49] and hypothesized, based on the structural similarity between azobenzenes and
- +-accelerated back-isomerization of Z-1. Reversible photoisomerization of phenylazotrimethylpyrazole 1. Supporting Information The Supporting Information features further experimental details, including synthesis and characterization of arylazopyrazole 1, characterization of inclusion complexes (E-1)22 and (Z
Morphology-tunable and pH-responsive supramolecular self-assemblies based on AB2-type host–guest-conjugated amphiphilic molecules for controlled drug delivery
Beilstein J. Org. Chem. 2019, 15, 1925–1932, doi:10.3762/bjoc.15.188

- utilized as host units to construct these stimuli-responsive supramolecular self-assemblies [21][22][23][24][25][26][27]. For example, β-CD can form inclusion complexes with guests such as azobenzene [28][29], ferrocene [30][31] and benzimidazole [32][33][34] to construct light-, redox-, and pH-responsive
- was adjusted to 5.0, accompanied with morphology transitions from FSSAs to spherical self-assemblies (SSAs) due to the pH-induced dissociation of β-CD/BM inclusion complexes (Scheme 1c,d), and the hydrophilic–hydrophobic interaction-induced formation of spherical micelles with BM units as inner
- SSAs in DCl/D2O due to the pH-induced dissociation of β-CD/BM inclusion complexes. In addition, the shift of the BM signals to lower field when the pH value was changed from neutral to acidic pH also confirmed the above result. On the basis of the abovementioned results, a possible morphology
Complexation of chiral amines by resorcin[4]arene sulfonic acids in polar media – circular dichroism and diffusion studies of chirality transfer and solvent dependence
Beilstein J. Org. Chem. 2019, 15, 1913–1924, doi:10.3762/bjoc.15.187
- chemistry. In the present study we demonstrate that resorcin[4]arene sulfonic acid (RSA) interacts with chiral amines (amino acid derivatives and aminocavitands) to form inclusion complexes and capsules based on electrostatic interactions. The complexes were characterized by circular dichroism and DOSY NMR
Synthesis of a [6]rotaxane with singly threaded γ-cyclodextrins as a single stereoisomer
Beilstein J. Org. Chem. 2019, 15, 1829–1837, doi:10.3762/bjoc.15.177

- -conjugated polymers [29][30][31]. On the other hand, γ-CD is relatively less employed in the synthesis of mechanically interlocked molecules despite of its ability to form interesting 1:2 inclusion complexes, and there are only few examples of rotaxane and catenane featuring γ-CD as an interlocked macrocycle
- orientation, possibly due to inter-ring interactions with the CB[6], to give the [6]rotaxane as one single stereoisomer. Considering the ability of γ-CD to form stable 1:2 inclusion complexes, these singly threaded [n]rotaxanes could serve as an entry point to other high order interlocked structures by
Host–guest interactions in nor-seco-cucurbit[10]uril: novel guest-dependent molecular recognition and stereoisomerism
Beilstein J. Org. Chem. 2019, 15, 1705–1711, doi:10.3762/bjoc.15.166
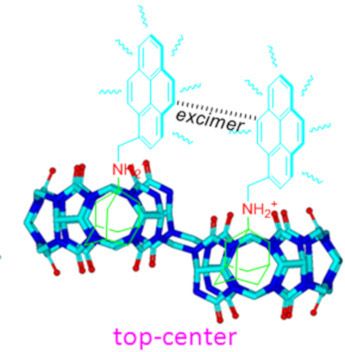
- and TΔS = −21.66 kJ·mol−1 for inclusion complex G1·host-1; ΔH = −65.77 kJ·mol−1 and TΔS = −26.46 kJ·mol−1 for the inclusion complex G2·host-1; ΔH = −39.01 kJ·mol−1 and TΔS = −1.02 kJ·mol−1 for the inclusion complex G1·host-2); while only the formation of the inclusion complexes of host-2 with G2 is
Water inside β-cyclodextrin cavity: amount, stability and mechanism of binding
Beilstein J. Org. Chem. 2019, 15, 1592–1600, doi:10.3762/bjoc.15.163

- .15.163 Abstract Cyclodextrins (CDs) are native host systems with inherent ability to form inclusion complexes with various molecular entities, mostly hydrophobic substances. Host cyclodextrins are accommodative to water molecules as well and contain water in the native state. For β-cyclodextrin (β-CD
- ; hydration; macrocycles; thermodynamic characteristics; Introduction Cyclodextrins (CDs), a family of enzymatically modified starches, are widely used as host macrocycles in forming inclusion complexes with various molecular entities of interest to food industry, pharmacology, cosmetics, catalysis, and
Reversible end-to-end assembly of selectively functionalized gold nanorods by light-responsive arylazopyrazole–cyclodextrin interaction
Beilstein J. Org. Chem. 2019, 15, 1407–1415, doi:10.3762/bjoc.15.140

- azobenzenes that form inclusion complexes with α- or β-CD exclusively in the trans configuration, not in the cis configuration [26]. This light-responsive interaction has been recently applied by Ma et al. for the end-to-end assembly of AuNR [27]. However, the system showed some limitations as the assembly
Complexation of a guanidinium-modified calixarene with diverse dyes and investigation of the corresponding photophysical response
Beilstein J. Org. Chem. 2019, 15, 1394–1406, doi:10.3762/bjoc.15.139

- employed (Table 1), indicating its privileged ability to form stable inclusion complexes with a variety of guest molecules. As for Fl, EY, RB, TPPS, AlPcS4 and TPS, a drastic complexation-induced fluorescence quenching without wavelength shifting was observed, which was assumed as the PET mechanism. 2,6
Selective detection of DABCO using a supramolecular interconversion as fluorescence reporter
Beilstein J. Org. Chem. 2019, 15, 1371–1378, doi:10.3762/bjoc.15.137
- porphyrin edge capsules to cone-shaped inclusion complexes depending on the presence of C60/C70, however, a process that was not selective for one of the guests [24]. A spectacular case of guest sensing, but not guest-induced recognition, was demonstrated by Clever in a supramolecular cage-to-cage
Efficient resolution of racemic crown-shaped cyclotriveratrylene derivatives and isolation and characterization of the intermediate saddle isomer
Beilstein J. Org. Chem. 2019, 15, 1339–1346, doi:10.3762/bjoc.15.133

- are Xe inclusion complexes that are of special interest as they help to establish laser-polarized 129Xe NMR spectroscopy for the imaging in biological systems [22][23][24][25]. For the formation of Xe cryptophane complexes, cryptophane-1.1.1 has proven to be a very suitable host as it still shows the



































































































































































































































































